CTSC | Stenoparib and COVID-19
Senior Scientists
Experienced COVID-9 researchers testing coronavirus inhibitors.
Abstract
Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerase (PARP), blocks replication of the SARS-CoV-2 coronaviruses in vitro.
By late 2020, the coronavirus disease (COVID-19) pandemic, caused by SARS-CoV-2 has caused tens of millions of infections and over 1 million deaths worldwide. A protective vaccine and more effective therapeutics are urgently needed. We evaluated a new PARP inhibitor, stenoparib, that recently advanced to Phase II clinical trials for treatment of ovarian cancer, for activity against human respiratory coronaviruses, including SARS-CoV-2, in vitro. Stenoparib exhibits dose-dependent suppression of SARS-CoV-2 multiplication and spread in Vero E6 monkey kidney and Calu-3 human lung adenocarcinoma cells. Stenoparib was also strongly inhibitory to the HCoV-NL63 human seasonal respiratory coronavirus. Compared to remdesivir, which inhibits viral replication downstream of cell entry, stenoparib impedes entry and post-entry processes as determined by time-of-addition (TOA) experiments. Moreover, a 10 mM dosage of stenoparib – below the approximated 25.5 mM half-maximally effective concentration (EC50), combined with 0.5 mM remdesivir suppressed coronavirus growth by more than 90%, indicating a potentially synergistic effect for this drug combination. Stenoparib as a standalone or as part of combinatorial therapy with remdesivir should be a valuable addition to the arsenal against COVID-19.

Fig. S1. Stenoparib inhibits plaque formation in Calu-3 cells.
Abstract Importance
Stenoparib impedes virus entry and post-entry processes, an advantage over drugs that have a narrower target range.
New therapeutics are urgently needed in the fight against COVID-19. Repurposing drugs that are either already approved for human use or are in advanced stages of the approval process can facilitate more rapid advances toward this goal. The PARP inhibitor stenoparib may be such a drug, as it is currently in Phase II clinical trials for the treatment of ovarian cancer and its safety and dosage in humans has already been established. Our results indicate that stenoparib possesses strong antiviral activity against SARS-CoV-2 and other coronaviruses in vitro. This activity appears to be based on multiple modes of action, where both pre-entry and post-entry viral replication processes are impeded. This may provide a therapeutic advantage over many current options that have a narrower target range. Moreover, our results suggest that stenoparib and remdesivir in combination may be especially potent against coronavirus infection.
Figures
Our data definitively shows that Stenoparib effectively inhibits SARS-CoV-2 in vitro.
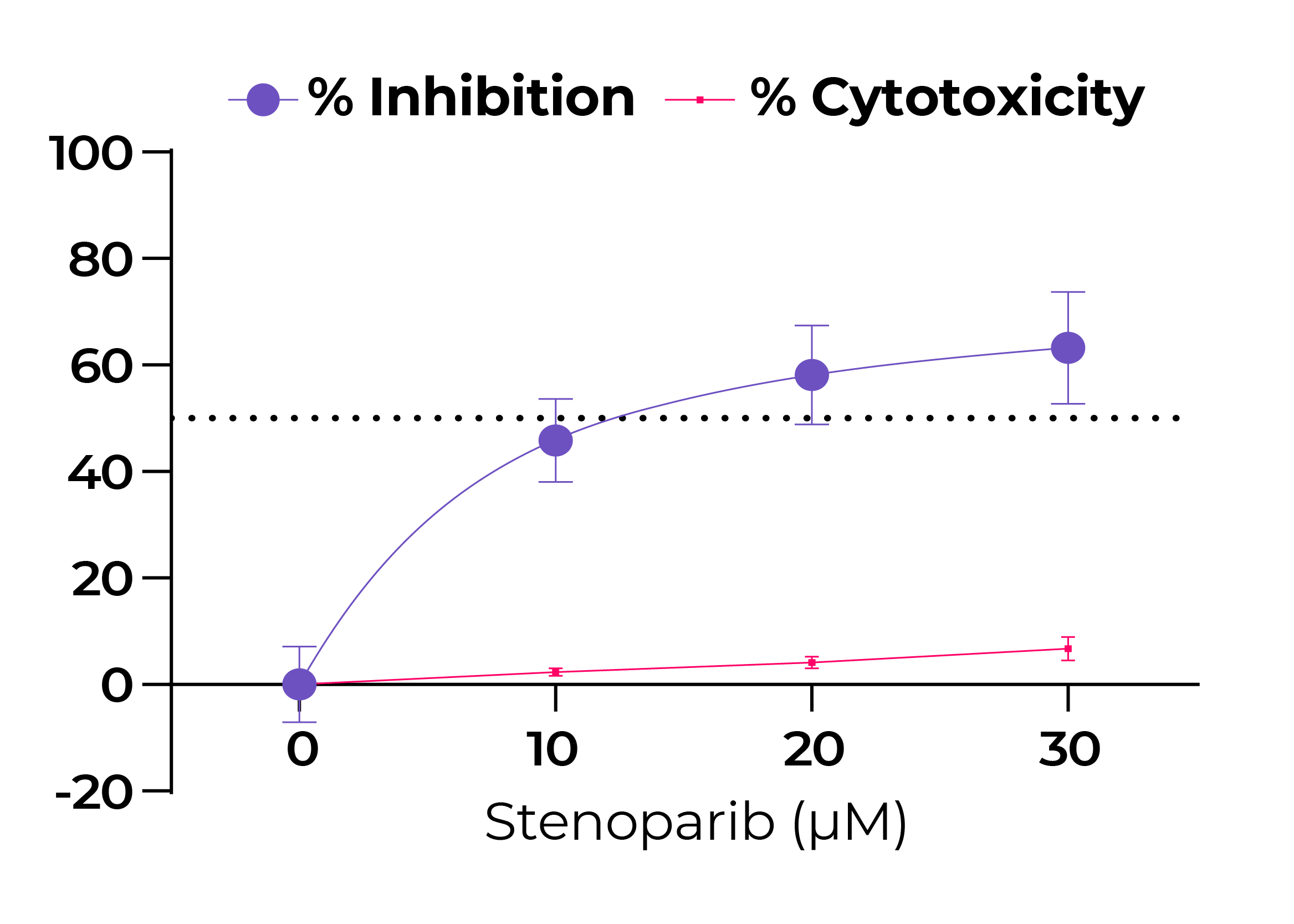
Fig. 1. Stenoparib exhibits dose-dependent inhibition of SARS-CoV-2 as measured by RT-qPCR.
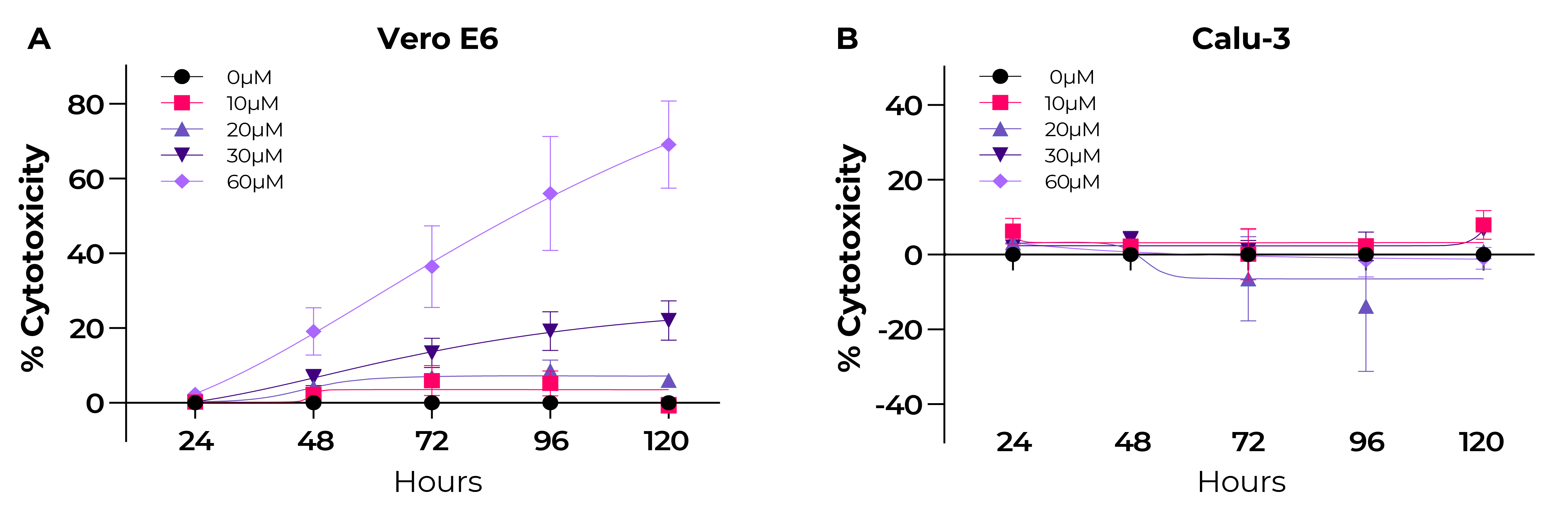
Fig. 2. Stenoparib is cytotoxic in Vero E6 cells at concentrations greater than 30 µM, but not in Calu-3 cells.
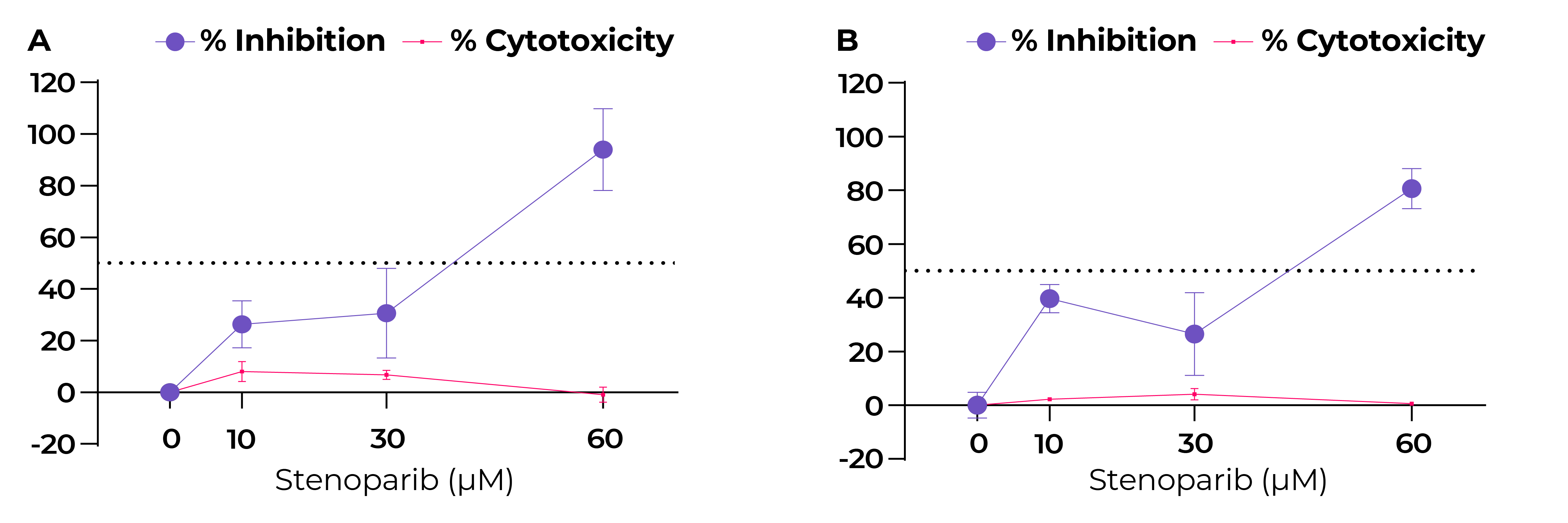
Fig. 3. Stenoparib exhibits dose-dependent inhibition of SARS-CoV-2 in Calu-3 cells.
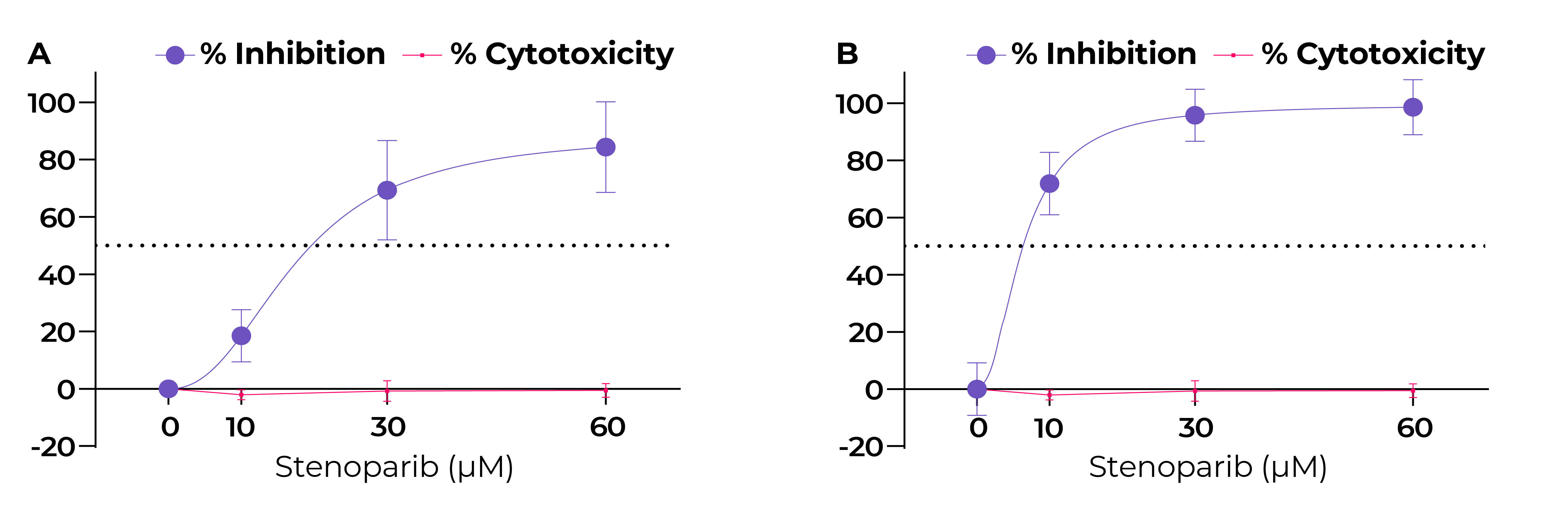
Fig. 4. Stenoparib exhibits dose-dependent inhibition of HCoV-NL63 in LLC-MK2 cells.
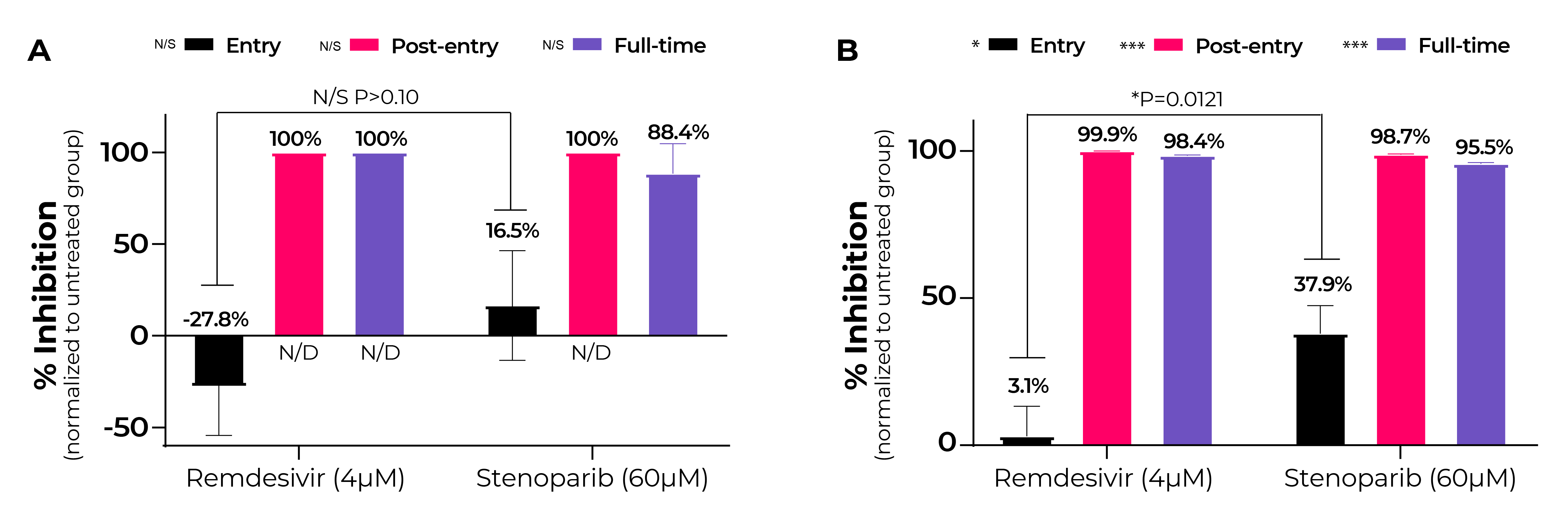
Fig. 5. Stenoparib inhibits HCoV-NL63 entry and post entry events while remdesivir inhibits post-entry.
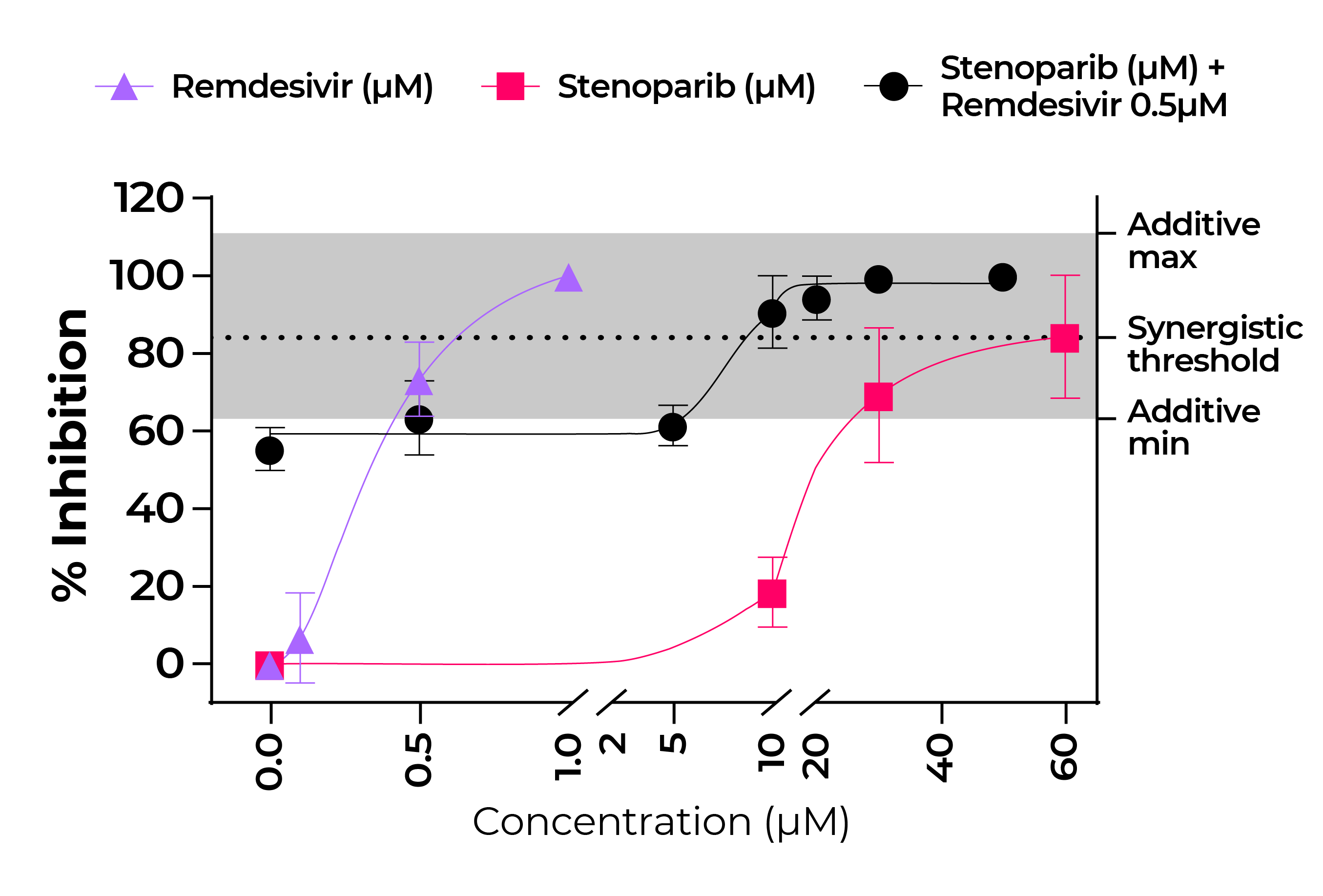
Fig. 6. Stenoparib and remdesivir in combination is a potent inhibitor of NL63.


















